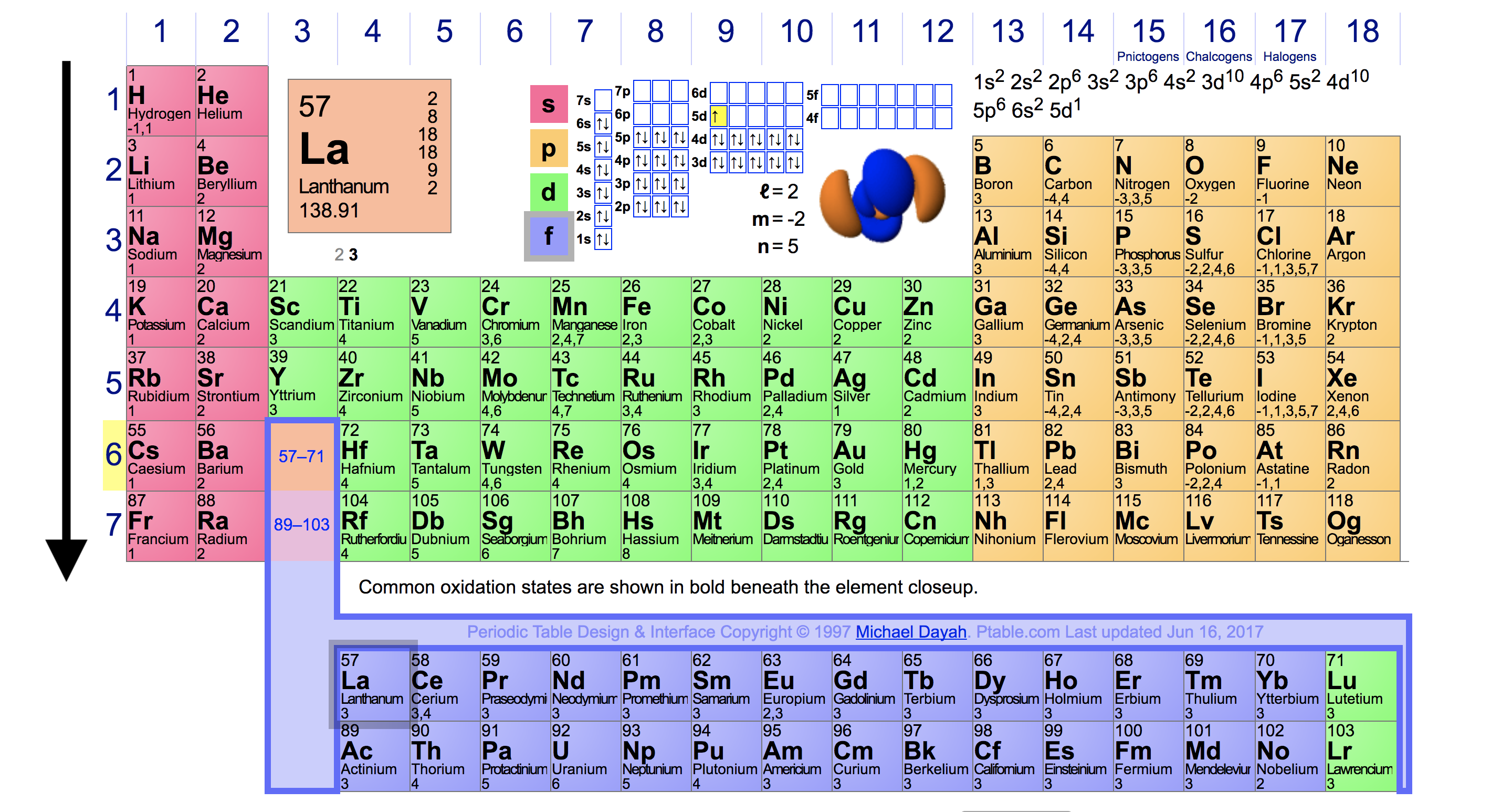
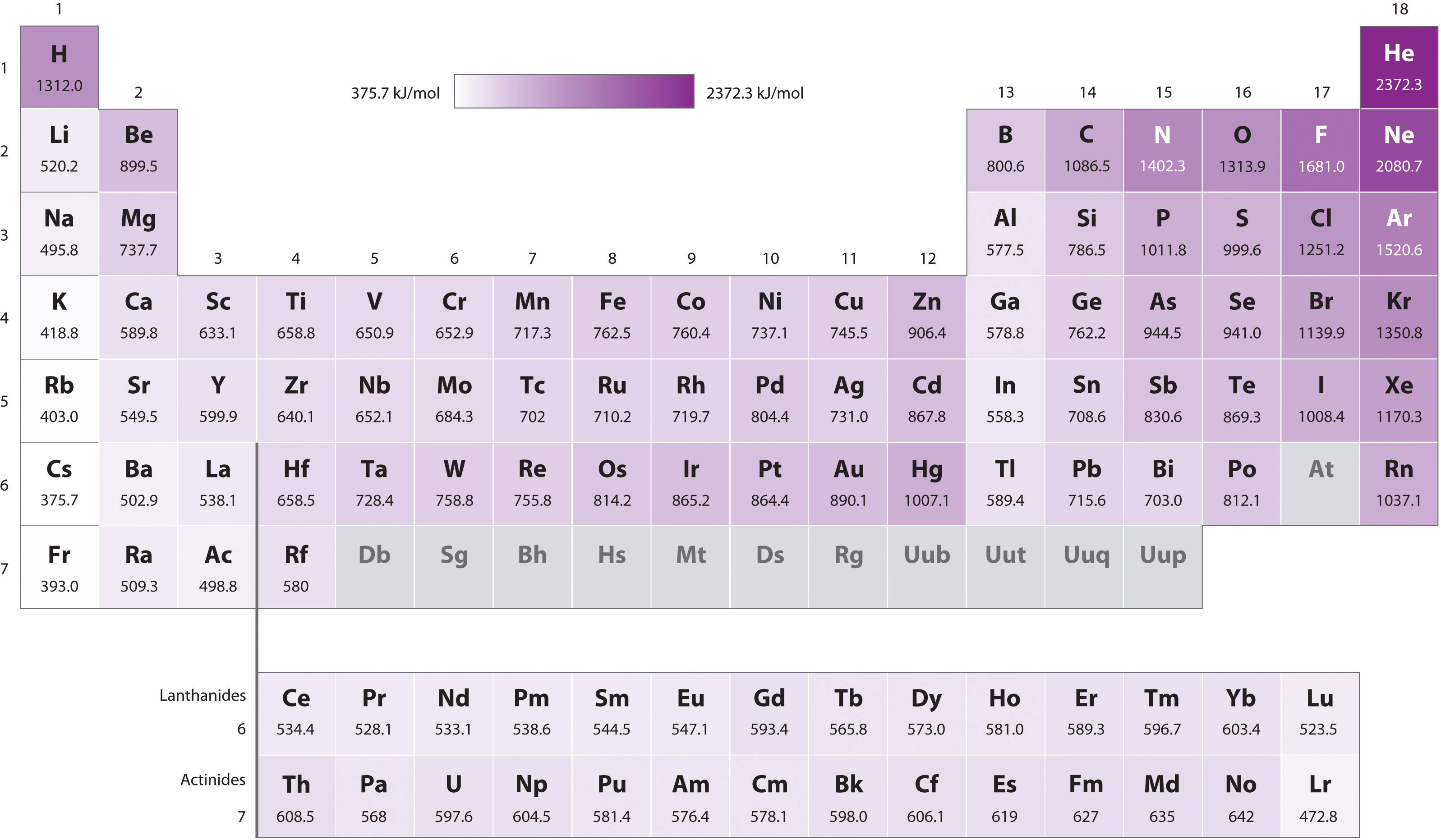
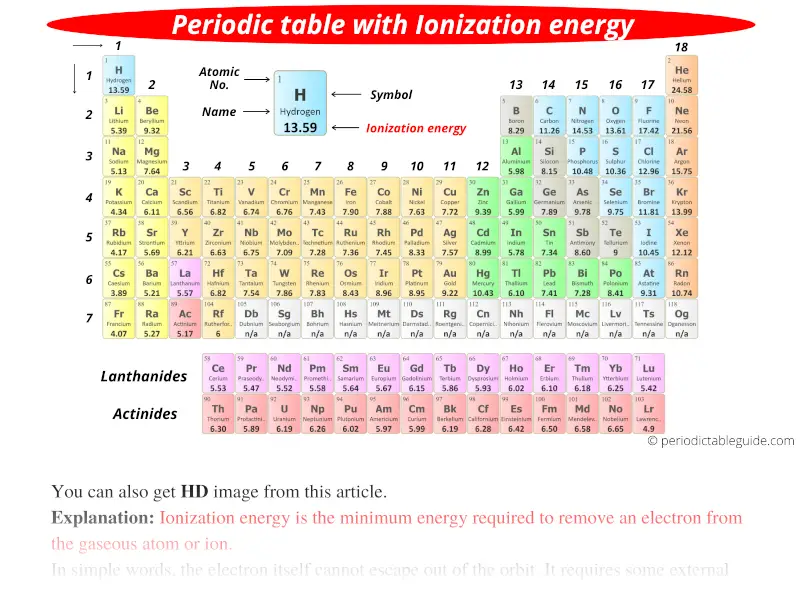
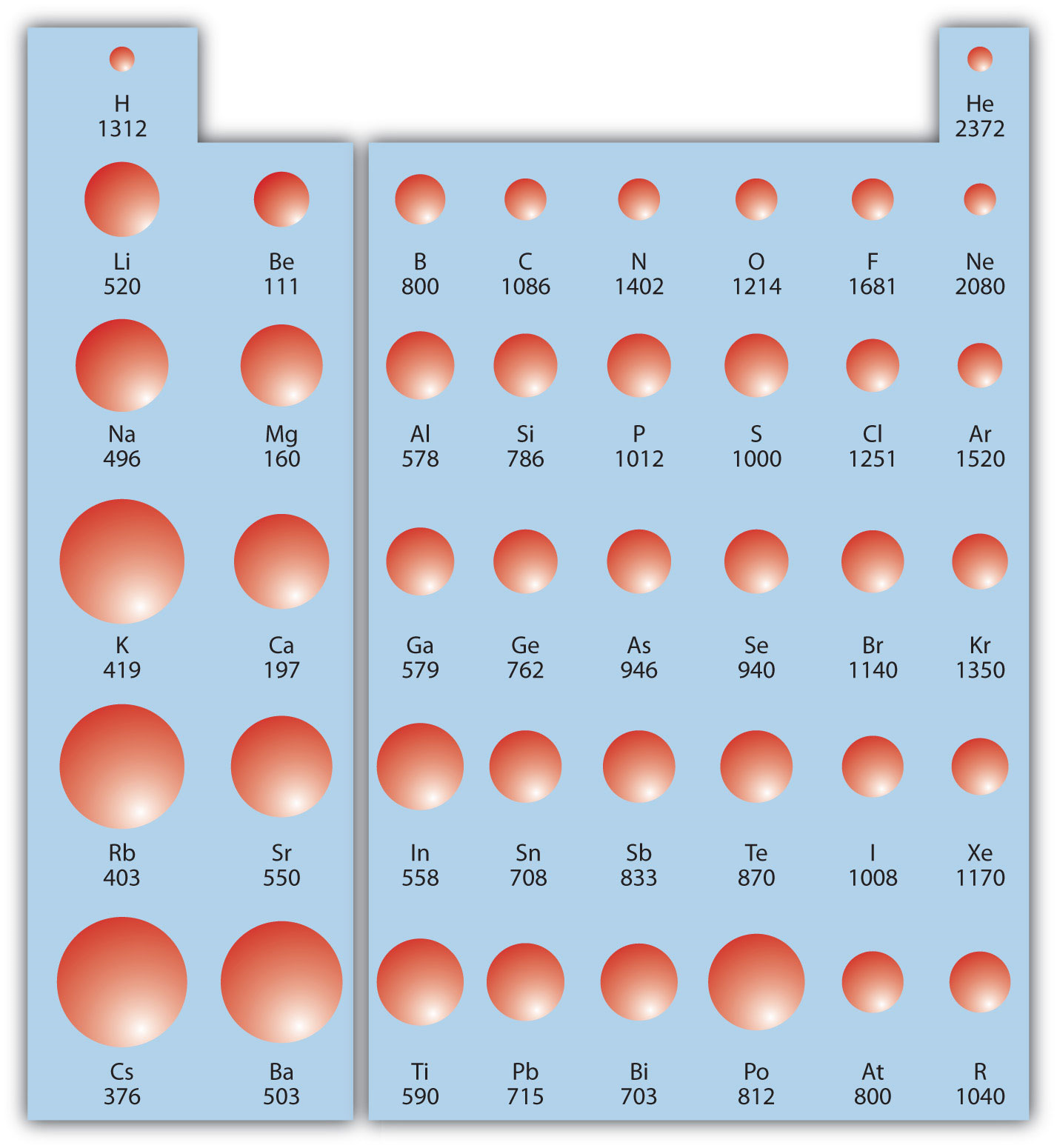
Ionic Energy Chart
Ionic Energy Chart - Web molar ionization energies of the elements. Web ionization energy is the quantity of energy that an isolated, gaseous atom in the ground electronic state must absorb to discharge an electron, resulting in a cation. Another is when each of 3 p orbitals have one. Web the values mentioned in the above periodic table is the first ionization energy and are given in electron volts (ev). The most common units of ionization energy are. Web magnesium is one of the most abundant minerals in your body. The stronger an electron is bound to an atom the more. Web ionization is the process of removing an electron from a neutral atom (or compound). Web ionization energy is a measure of the energy needed to pull a particular electron away from the attraction of the nucleus. Web for each atom, the column marked 1 is the first ionization energy to ionize the neutral atom, the column marked 2 is the second ionization energy to remove a second electron. A high value of ionization energy. Web when electrons are removed in succession from an element, the transition from removing valence electrons to removing core electrons results in a large jump in ionization. Hundreds of chemical reactions in your body involve magnesium. Web there are couple of reasons for that. Web ionization energy chart of all the elements is given below. The energy required to remove an electron is the ionization energy. One is that when electrons start to fill p orbital the ionization energy goes down a little. Web for each atom, the column marked 1 is the first ionization energy to ionize the neutral atom, the column marked 2 is the second ionization energy to remove a second electron. The stronger an electron is bound to an atom the more. Web ionization energy, in chemistry and physics, the amount of energy required to remove an electron from an isolated atom or molecule. Web the periodic table trend on a graph. The stronger an electron is bound to an atom the more. Web ionization energy, in chemistry and physics, the amount of energy required to remove an electron from an isolated atom or molecule. Web the periodic table of the elements (with ionization energies) element name. Web ionization energy is the energy required. This is the energy per mole necessary to remove. Web ionization energy, in chemistry and physics, the amount of energy required to remove an electron from an isolated atom or molecule. The stronger an electron is bound to an atom the more. Ionization energy is always positive. A high value of ionization energy. Web ionization energy is a measure of the energy needed to pull a particular electron away from the attraction of the nucleus. Your body needs it for muscle. The ionization energy associated with. Web for each atom, the column marked 1 is the first ionization energy to ionize the neutral atom, the column marked 2 is the second ionization energy. Ionization energy is always positive. Web ionization energy, in chemistry and physics, the amount of energy required to remove an electron from an isolated atom or molecule. The most common units of ionization energy are. Web ionization energy is the quantity of energy that an isolated, gaseous atom in the ground electronic state must absorb to discharge an electron, resulting. First ionization energy, second ionization energy as well as third ionization energy of the elements are. Ionization energy is always positive. Web for each atom, the column marked 1 is the first ionization energy to ionize the neutral atom, the column marked 2 is the second ionization energy to remove a second electron. This is the energy per mole necessary. The energy required to remove the outermost electron from an atom or a positive ion in its ground level. Web ionization energy is the energy required to remove an electron from a neutral atom in its gaseous phase. Web ionization energy is the amount of energy needed to remove an electron from a neutral gaseous atom and form an ion.. Web when electrons are removed in succession from an element, the transition from removing valence electrons to removing core electrons results in a large jump in ionization. Web molar ionization energies of the elements. The ionization energy associated with. Web looking at the graph of ionization energies, it is clear that indium(atomic number 49) does have a lower ionization energy. Web explore how ionization energy changes with atomic number in the periodic table of elements via interactive plots. Web ionization energy is the minimum energy required to remove an electron from an atom or ion in the gas phase. One is that when electrons start to fill p orbital the ionization energy goes down a little. Web ionization energy is. Web ionization energy is the energy required to remove an electron from a neutral atom in its gaseous phase. Web the values mentioned in the above periodic table is the first ionization energy and are given in electron volts (ev). Web magnesium is one of the most abundant minerals in your body. On the periodic table, first. Web there are. Web when electrons are removed in succession from an element, the transition from removing valence electrons to removing core electrons results in a large jump in ionization. Ionization energy is always positive. A high value of ionization energy. Your body needs it for muscle. On the periodic table, first. A high value of ionization energy. The energy required to remove the outermost electron from an atom or a positive ion in its ground level. Web ionization energy is a measure of the energy needed to pull a particular electron away from the attraction of the nucleus. This is the energy per mole necessary to remove. Web ionization energy is the energy required to remove an electron from a neutral atom in its gaseous phase. Web magnesium is one of the most abundant minerals in your body. Web the values mentioned in the above periodic table is the first ionization energy and are given in electron volts (ev). Web when electrons are removed in succession from an element, the transition from removing valence electrons to removing core electrons results in a large jump in ionization. Web chemists define the ionization energy (\(i\)) of an element as the amount of energy needed to remove an electron from the gaseous atom \(e\) in its ground state. The most common units of ionization energy are. The table lists only the first ie in ev units. Web for each atom, the column marked 1 is the first ionization energy to ionize the neutral atom, the column marked 2 is the second ionization energy to remove a second electron. Web an element's first ionization energy is the energy required to remove the outermost, or least bound, electron from a neutral atom of the element. Web the ionization energy of atoms, denoted e i, is measured by finding the minimal energy of light quanta or electrons accelerated to a known energy that will kick out the least bound. One is that when electrons start to fill p orbital the ionization energy goes down a little. Web ionization energy is the minimum energy required to remove an electron from an atom or ion in the gas phase.Ionization Enthalpy NEET Lab
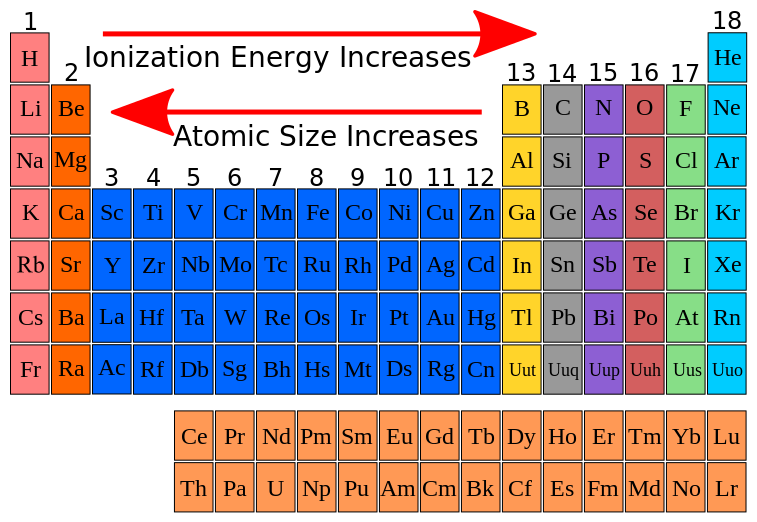
FileIonization energy atomic size.svg Wikimedia Commons
Example
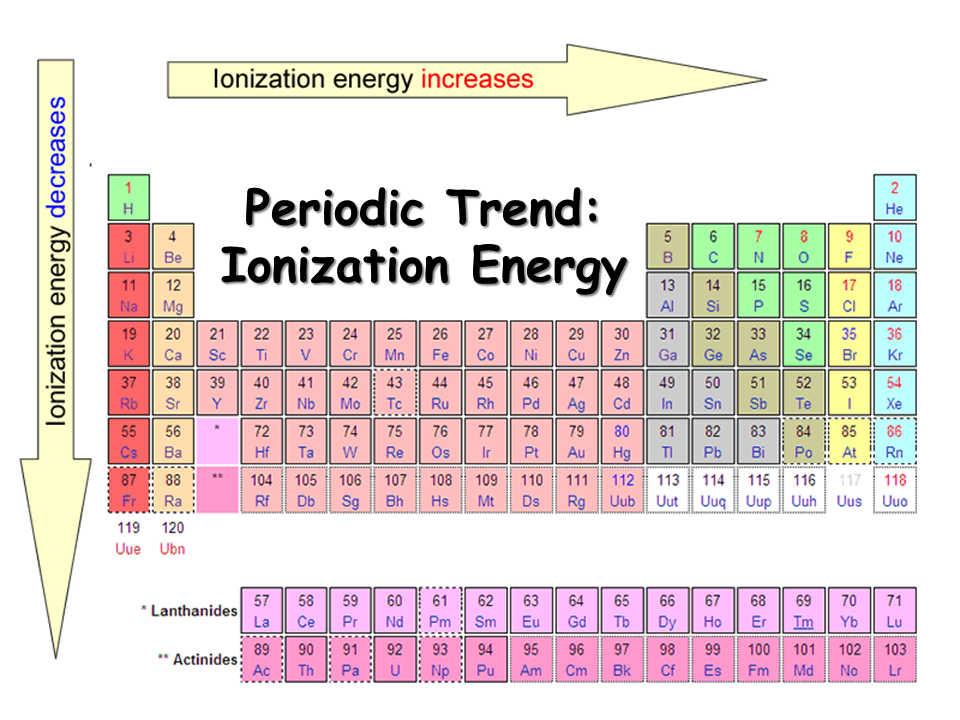
Periodic Table Ionization Energy Chart
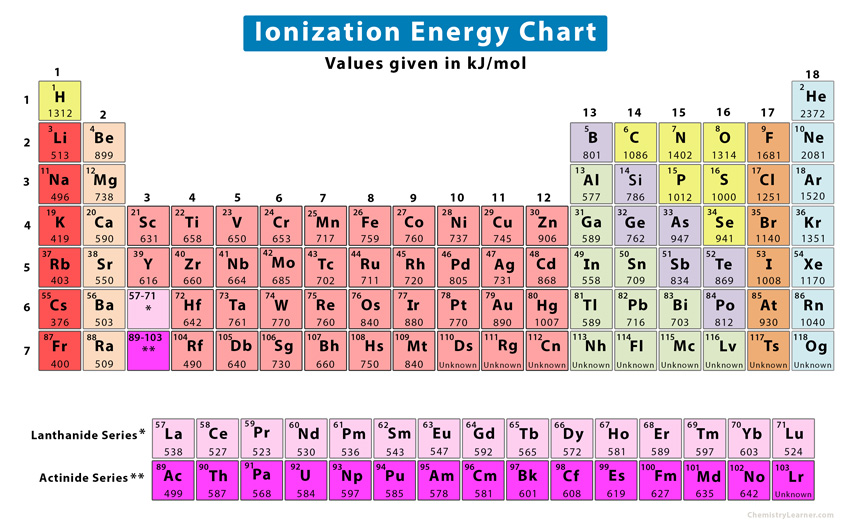
Ionization Energy Definition, Chart & Periodic Table Trend
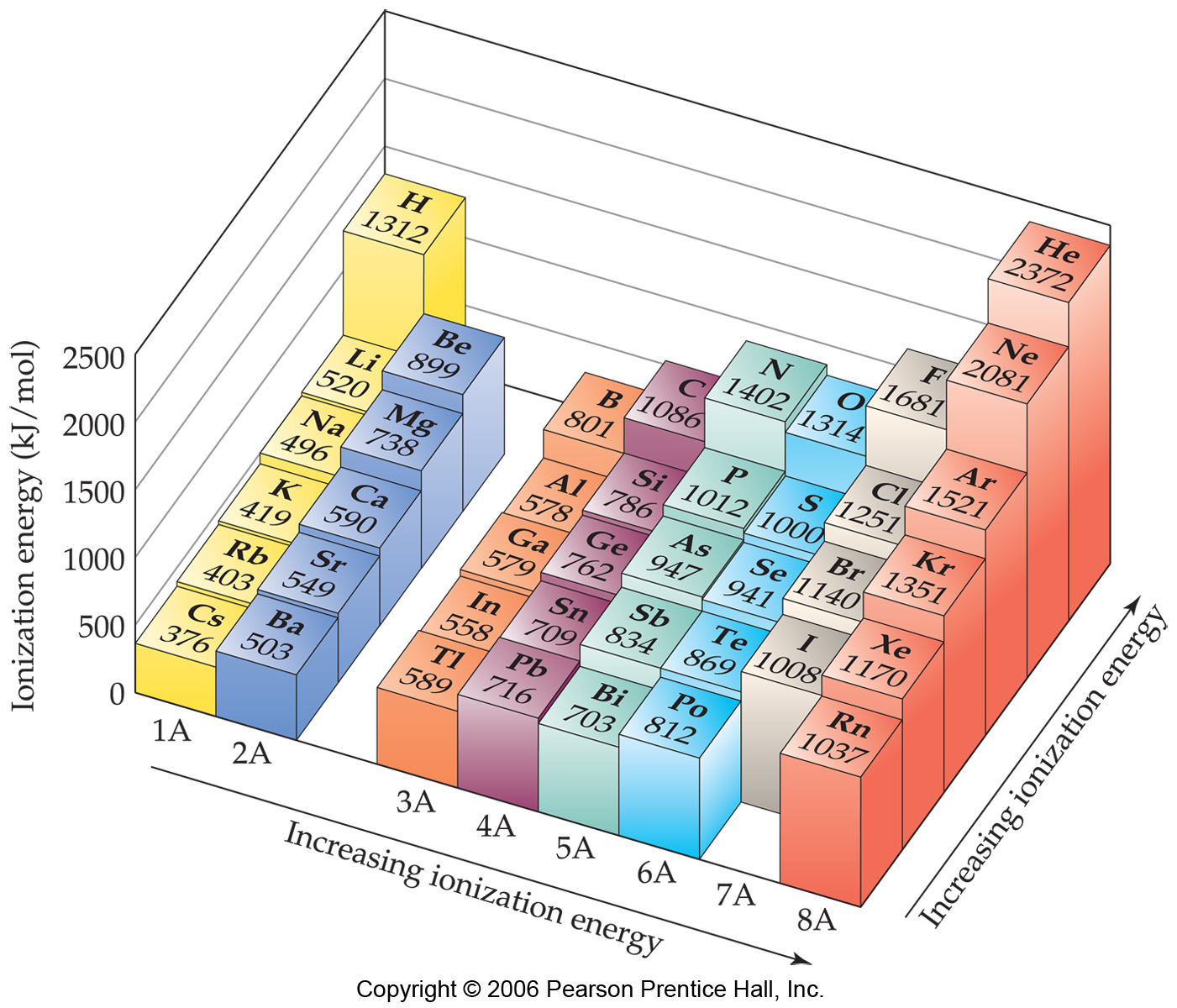
Periodic Trends in Ionization Energy Chemistry Socratic
Periodic Table Ionization Energy Labeled
Periodic table with Ionization Energy Values (Labeled Image)
What Is Ionization Energy? Definition and Trend
9.9 Periodic Trends Atomic Size, Ionization Energy, and Metallic
Web The Periodic Table Of The Elements (With Ionization Energies) Element Name.
The Ionization Energy Associated With.
Web Ionization Is The Process Of Removing An Electron From A Neutral Atom (Or Compound).
The Stronger An Electron Is Bound To An Atom The More.
Related Post: