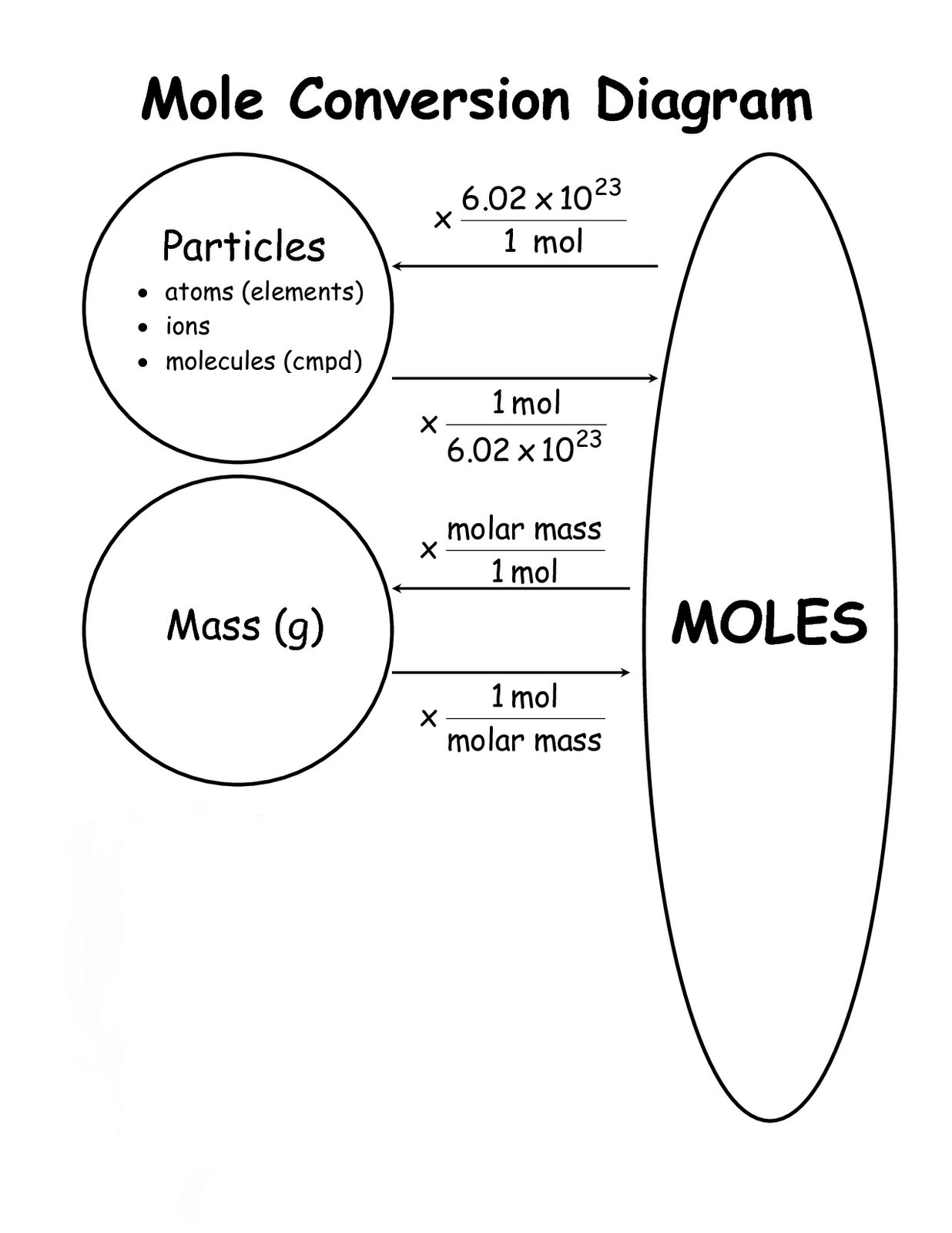
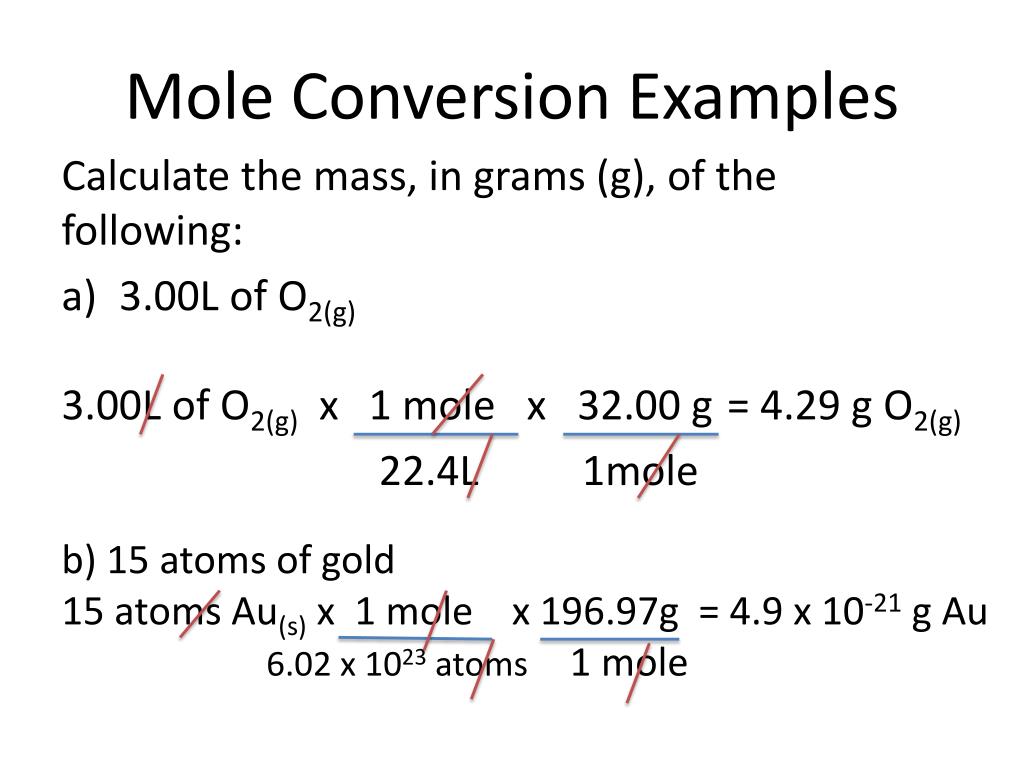
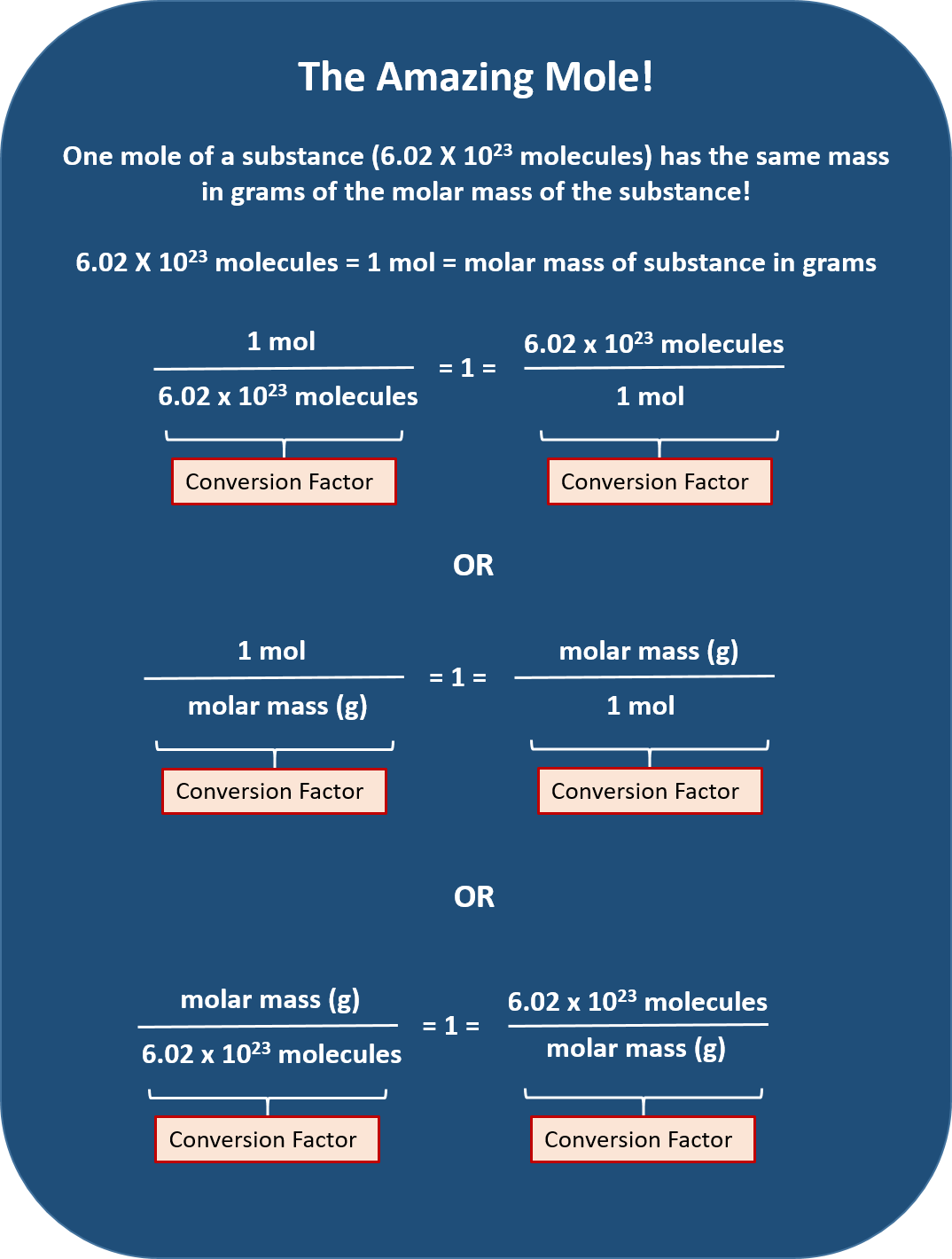
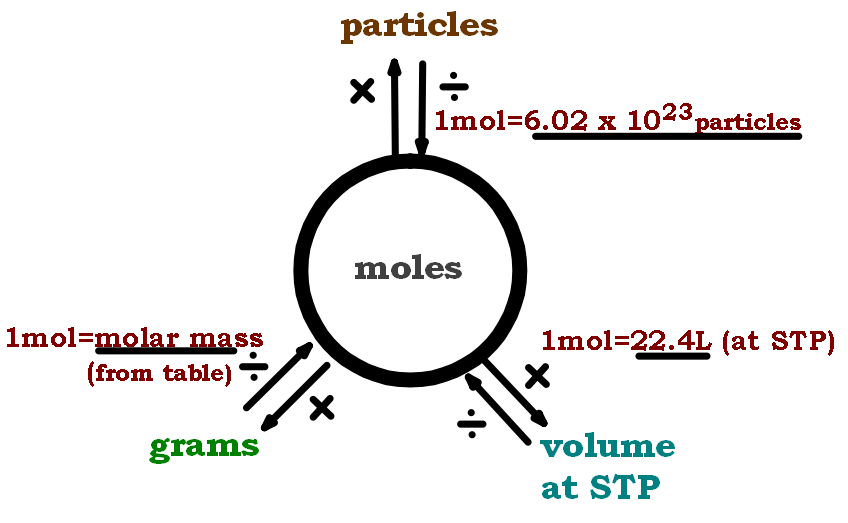
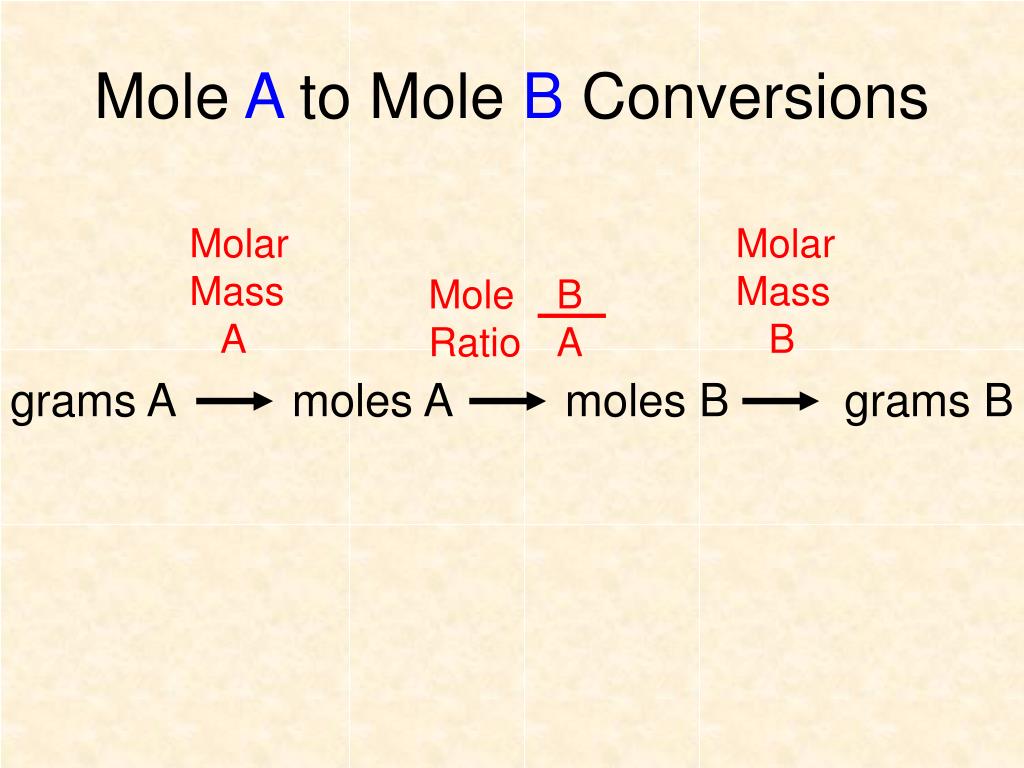
Moles Conversion Chart
Moles Conversion Chart - Using our unit conversion techniques, we can use the mole label to convert back and forth between the number of particles and moles. Our atoms to moles calculator easily converts atoms or molecules to moles. If you are struggling, it will quickly help you understand how to convert from moles, to grams, liters, molecules, and back. The element carbon exists in. From mass (grams) to moles: Web conversions between moles and number of particles. Web mole conversion chart national mole day october 23. Web perform conversions between mass and moles of a substance. Web whether you’re converting from moles to grams, moles to volume, or moles to particles (atoms or molecules), use this quick guide to remind you of how to do each type of mole conversion: One mole is equal to 6.022 x 10^23 atoms, molecules or other particles. Web the mole map is a powerful tool to visualize mole conversions. Each set of units must be converted to moles first. If you are struggling, it will quickly help you understand how to convert from moles, to grams, liters, molecules, and back. The element carbon exists in. Divide your initial mass by the molar mass of the compound as determined by the periodic table. The study of the numerical relationships between the reactants and the products in balanced chemical reactions is called stoichiometry. Web a mole road map is a simple guide for converting between units of mass, volume, and number of particles. Web conversions between moles and number of particles. From mass (grams) to moles: Web mole conversion chart national mole day october 23. If you are struggling, it will quickly help you understand how to convert from moles, to grams, liters, molecules, and back. Web conversions between moles and number of particles. Our atoms to moles calculator easily converts atoms or molecules to moles. Web moles are a type of unit conversion used in chemistry to measure the amount of substances. Using our. Web we can use these ratios to determine what amount of a substance, in moles, will react with or produce a given number of moles of a different substance. From mass (grams) to moles: Using our unit conversion techniques, we can use the mole label to convert back and forth between the number of particles and moles. One mole is. One mole is equal to 6.022 x 10^23 atoms, molecules or other particles. Divide your initial mass by the molar mass of the compound as determined by the periodic table. Web perform conversions between mass and moles of a substance. If you are struggling, it will quickly help you understand how to convert from moles, to grams, liters, molecules, and. Web moles are a type of unit conversion used in chemistry to measure the amount of substances. One mole is equal to 6.022 x 10^23 atoms, molecules or other particles. Web the quantity of substance in moles is equal to the number of molecules divided by the avogadro constant (6.02214076 × 10 23). Measure the mass of your sample in. Use a balanced chemical equation to determine molar. Divide your initial mass by the molar mass of the compound as determined by the periodic table. Web perform conversions between mass and moles of a substance. Web a mole road map is a simple guide for converting between units of mass, volume, and number of particles. The study of the numerical. Use a balanced chemical equation to determine molar. The element carbon exists in. Web the mole map is a powerful tool to visualize mole conversions. From mass (grams) to moles: Web moles are a type of unit conversion used in chemistry to measure the amount of substances. Our atoms to moles calculator easily converts atoms or molecules to moles. The study of the numerical relationships between the reactants and the products in balanced chemical reactions is called stoichiometry. Web we can use these ratios to determine what amount of a substance, in moles, will react with or produce a given number of moles of a different substance.. Web the mole map is a powerful tool to visualize mole conversions. Web the quantity of substance in moles is equal to the number of molecules divided by the avogadro constant (6.02214076 × 10 23). Using our unit conversion techniques, we can use the mole label to convert back and forth between the number of particles and moles. Divide your. Web the quantity of substance in moles is equal to the number of molecules divided by the avogadro constant (6.02214076 × 10 23). Divide the mass by the molar mass to find the number of moles in your sample. Divide your initial mass by the molar mass of the compound as determined by the periodic table. Web the mole map. Web we can use these ratios to determine what amount of a substance, in moles, will react with or produce a given number of moles of a different substance. By converting from moles to grams, liters, and other units, chemists can calculate the mass or volume of a given substance. Use a balanced chemical equation to determine molar. Each set. Find the molar mass of the substance you are analyzing. Web perform conversions between mass and moles of a substance. Measure the mass of your sample in grams. One mole is equal to 6.022 x 10^23 atoms, molecules or other particles. Web a mole road map is a simple guide for converting between units of mass, volume, and number of particles. Divide the mass by the molar mass to find the number of moles in your sample. The element carbon exists in. Web the mole map is a powerful tool to visualize mole conversions. Our atoms to moles calculator easily converts atoms or molecules to moles. Divide your initial mass by the molar mass of the compound as determined by the periodic table. Each set of units must be converted to moles first. Web we can use these ratios to determine what amount of a substance, in moles, will react with or produce a given number of moles of a different substance. Web to convert from grams to moles, follow these few simple steps: The study of the numerical relationships between the reactants and the products in balanced chemical reactions is called stoichiometry. Convert from mass or moles of one substance to mass or moles of another substance in a chemical reaction. From mass (grams) to moles:Mole Calculation O Level Secondary Chemistry Tuition
Molar Mass Conversion Chart
Mole Conversion Chart Basicand Advanced Conversions1 REF Mole
The Mole Presentation Chemistry
Chemistry Mysteries Mole Conversions
PPT Chemistry 20 Mole Conversions PowerPoint Presentation, free
Moles to Grams Conversion Examples
Chapter 6 Quantities in Chemical Reactions Chemistry
Unit 7 Math of Chemistry Ivy Way Science
PPT Chapter 12 Stoichiometry PowerPoint Presentation, free download
By Converting From Moles To Grams, Liters, And Other Units, Chemists Can Calculate The Mass Or Volume Of A Given Substance.
Web Conversions Between Moles And Number Of Particles.
Web The Quantity Of Substance In Moles Is Equal To The Number Of Molecules Divided By The Avogadro Constant (6.02214076 × 10 23).
Using Our Unit Conversion Techniques, We Can Use The Mole Label To Convert Back And Forth Between The Number Of Particles And Moles.
Related Post:


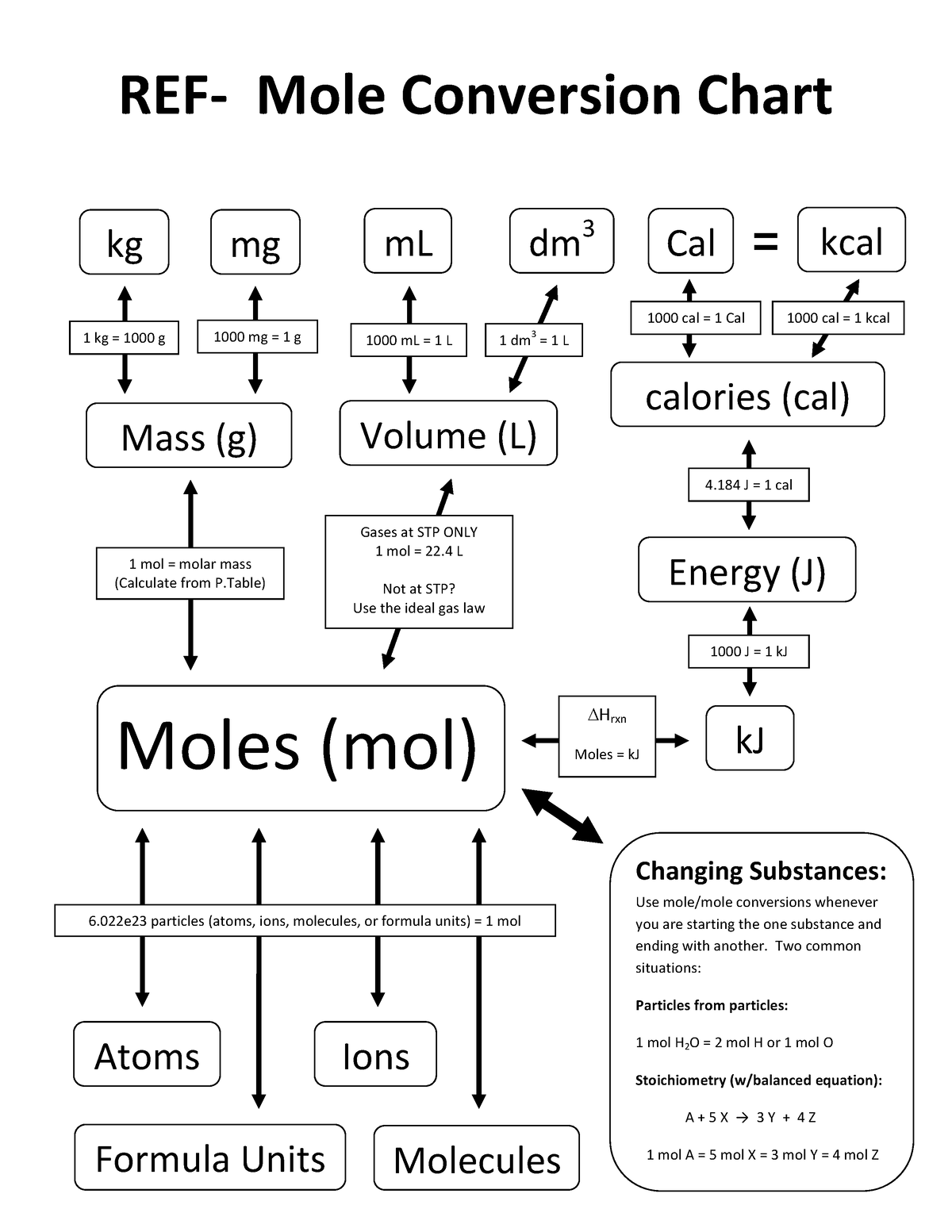
.PNG)